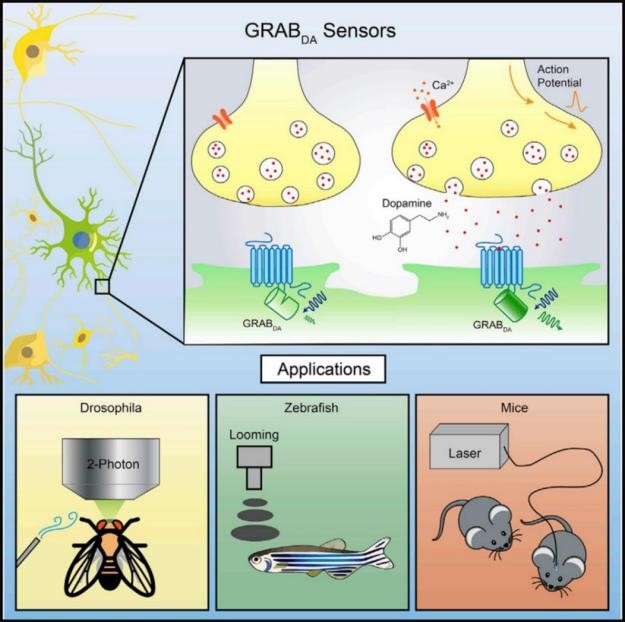
Li Lab has deposited sensor plasmids at BrainVTA for distribution to the research community, and all GRAB sensors (including the lastest ACh, DA, NE, 5-HT, Ado, ATP, VIP, CCK and eCB sensors) with AAV are available.
Please go to pre-made AAV center to search the published sensors, for un-published ones, please contact Li lab for permission fist!
Features of published sensors
| Name | Neurotransmitter | Version | Color | Backbones(From human) | Affinity | Signal Response Amplitude | Dynamics | Downstream Signal Coupling | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ACh2.0 | Acetylcholine | First generation | Green | M8 receptor | EC50=20nM | ΔF/F=90-200%; τon=200ms | τon~200ms, | Weak | [1] |
| ACh3.0 | Acetylcholine | Second generation | Green | M8 receptor | EC50=20nM | ΔF/F=60-100%; τon=200ms | τon~112ms,τoff~580ms | Hardly | [2] |
| ACh3.0-mut | Acetylcholine | Control of Second generation | Green | M8 receptor (W210A mutation) | EC50=20nM | ΔF/F=-0.18%; τon=200ms | / | Hardly | [2] |
| DA1m | Dopamine | First generation | Red | D2 receptor | EC50=3-10nM | ΔF/F=60-100%; τon=60ms, τoff=700ms | τon~60ms, | Hardly | [3] |
| DA1h | Dopamine | First generation | Red | D2 receptor | EC50=3-10nM | ΔF/F=80-150%; τon=60ms, τoff=250ms | τon~140ms, | Hardly | [3] |
| DA(D104A) | Dopamine | Control of First generation | Red | D2 receptor (D104A and S110R mutation) | EC50>500nM | No effect | / | [3] | |
| DA2m/DA4m | Dopamine | Second generation | Green/Red | D2 receptor | EC50=3-10nM | ΔF/F=30-70%; τon=80ms, τoff=100ms | τon~40ms, | Little | [4] |
| DA2h/DA4h | Dopamine | Second generation | Green/Red | D2 receptor | EC50=3-10nM | ΔF/F=80-120%; τon=70ms, τoff=200ms | τon~50ms, | Little | [4] |
| DA(D104A2nd) | Dopamine | Control of Second generation | Green/Red | D2 receptor (D104A and S110R mutation) | EC50>500nM | No effect | / | [4] | |
| αDA1m (αDA2.5m) | Dopamine | First generation | Red | D2 receptor | EC50=3-10nM | ΔF/F=150-200%; τon=30ms, τoff=80ms | τon~80ms, | Little | [4] |
| αDA1h (αDA2.5h) | Dopamine | First generation | Red | D2 receptor | EC50=3-10nM | ΔF/F=100-150%; τon=30ms, τoff=200ms | τon~60ms, | Little | [4] |
| αDA(D104A2nd) | Dopamine | Control of Second generation | Red | D2 receptor (D104A and S110R mutation) | EC50>500nM | No effect | / | [4] | |
| NE1m(β2.1) | Norepinephrine | First generation | Green | α2A receptor | EC50=120nM | ΔF/F=200%; τon=30ms, τoff=70ms | τon ~70ms, | Uncoupled | [5] |
| NE1h(β2.2) | Norepinephrine | First generation | Green | α2A receptor | EC50=120nM | ΔF/F=130%; τon=30ms, τoff=200ms | τon ~30ms, | Uncoupled | [5] |
| NEmut | Norepinephrine | / | Green | α2A receptor | EC50~0uM | No effect | / | / |
|
| Ado1.0 | Adenosine | Control | Green | α2A receptor | EC50~60nM | ΔF/F0~130% | τon~36ms, | Hardly |
|
| Ado1.0mut | Adenosine | / | Green | α2A receptor | EC50~0uM | No effect | / | / |
|
| 5-HT1.0 | Serotonin | Control | Green | 5-HT2C receptor | EC50~22nM | ΔF/F0~250% | τon~0.2s, | Uncoupled |
|
| 5-HTmut | Serotonin | / | Green | 5-HT2C receptor | EC50~0uM | No effect | / | / |
|
FAQ:
1. What do the abbreviation h/m/l/mut mean, and what about the number?
h/m/l is the abbreviation of high/median/low, which indicates the sensors' apparent affinity towards transmitter. Most of the GRAB sensors have transmitter-insensitive mutant for comparison with GRAB sensors to check signal specificity. The number represents the version of sensor, the higher version, the better performance.
2. How to use GRAB sensors?
GRAB sensors are genetically encoded sensors for neurotransmitters, similar to other genetically encoded sensors (e.g. GCaMP). They could be expressed in specific cell-types and are able to detect dynamics of extracellular neurotransmitters in cultured cell, brain slice and living animal (e.g. fly, zebrafish, mice, rat, monkey etc.). For the aspect of instruments, GRAB sensors could perform well in epifluorescence microscopy, confocal microscopy, 2P microscopy and fiber photometry recording.
3. Can the sensors be expressed in vivo for a long-term period?
Yes. We can record good signals from GRAB sensor after expression for 3 to 6 months in mice brain by AAV infection.
4. Can GRAB sensors detect excitability of neurons?
No. The change of fluorescent intensity only indicates the dynamics of corresponding neurotransmitter, which has no direct correlation with excitability of neurons. Other approach is needed for detection of neuron excitability.
Reference
-
Jing, M., et al. (2018). "A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies." Nat Biotechnol 36(8): 726-737.
-
Jing, M., et al. (2020). "An optimized acetylcholine sensor for monitoring in vivo cholinergic activity." Nat Methods 17(11): 1139-1146.
-
Sun, F., et al. (2018). "A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice." Cell 174(2): 481-496 e419.
-
Sun, F., et al. (2020). "Next-generation GRAB sensors for monitoring dopaminergic activity in vivo." Nat Methods 17(11): 1156-1166.
-
Feng, J., et al. (2019). "A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine." Neuron 102(4): 745-761 e748.
-
Peng, W., et al. (2020). "Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons." Science 369(6508).
-
Wan, J., et al. (2021). "A genetically encoded sensor for measuring serotonin dynamics." Nat Neurosci.

