Cell Line Development Service Workflow
MicroRNA Service Workflow
| Project Discussion | Contract Confirmation and project initiation | Vector Construction | Lentivirus production | Lentivirus transduction and screening | Cell cryopreservation and QC | Delivery |
|---|---|---|---|---|---|---|
| Subcloning into appropriate vectors | High-efficiency lentivirus systems enable the stable integration of target genes into the host genome. | | Stable cell validation by FACS or WB | 1-3 single cell clones | ||
| Mycoplasma testing | Report |
Cell Line Development Technical Workflow
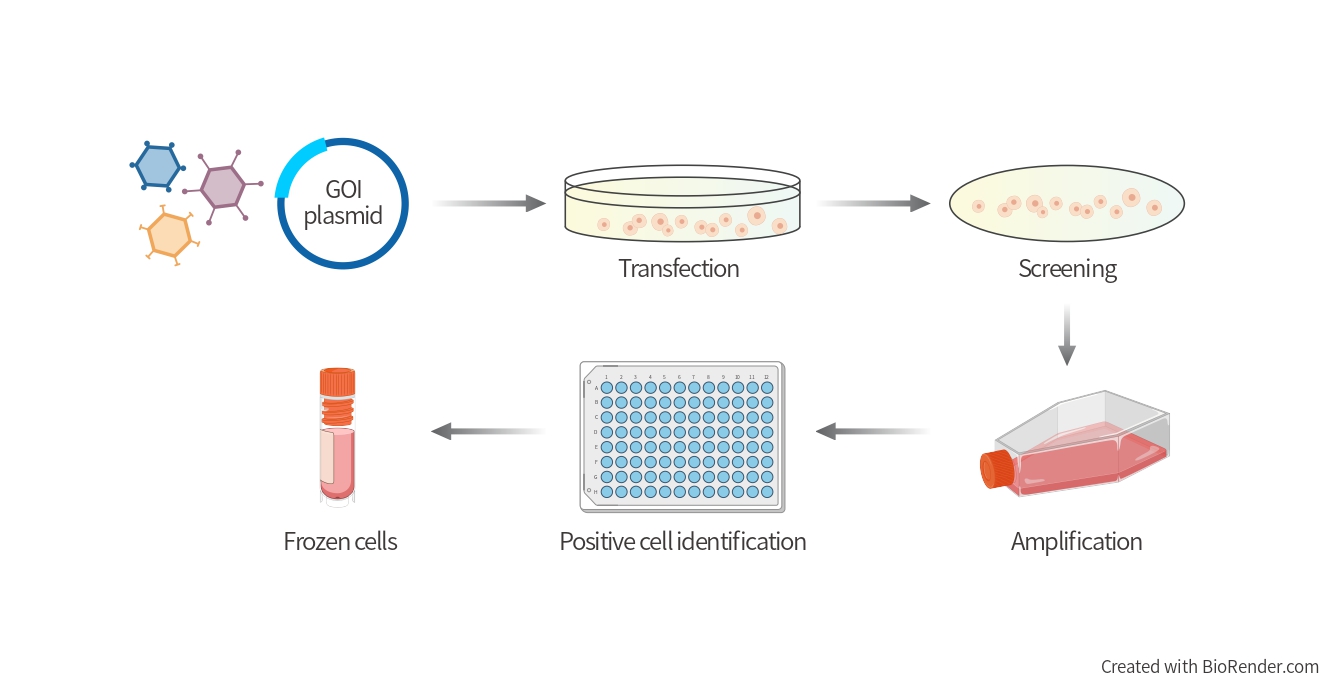
Why choose BrainVTA?
- BrainVTA have experience working with a wide variety of cell ranging from easy-to-transfect cell lines to hard-to-transfect cell lines.
- BrainVTA provide consultation services to discuss and evaluate the technical feasibility on the modification desired and the cell background. We take the time to understand your goals!
- Customized cell lines is diverse, including Knock-out, knock-down, knock-in, over-expression,
Online Inquiry for Stable Cell Line Generation
| Basic Research | Biologics Development |
|---|---|
| Stable cell lines offer the benefit of reduced inconsistencies and costs compared with transiently transfected cells. Stable cell lines developed by BrainVTA can decrease the loss of expression of exogenous gene fragments and help assess the long-term effects of target genes on cells. | A stable cell line can provide protein (or antibody) samples at well-characterized properties, which later helps functionally verify the toxicology of the biologic. From a quality control standpoint, ensuring that only single-cell clones are isolated from the selected pool is crucial. Our stable cell platform lays the foundation for the development of biologics. |
Stable Cell Line Development FAQs
-
1.Which bacterial strain does BrainVTA use for cloning its vectors? What bacterial strains can I use for propagating my plasmid obtained from BrainVTA?
Clients simply provide us with protein/gene sequences or NCBI #. According to the customers' specific research needs, our scientists will design stable cell lines and establish purification strategies.
-
2. How can I select between monoclonal cell lines and Polyclonal cell lines ?
Polyclonal cells can meet most experimental needs, but if your experiments require a high degree of consistency and reproducibility(Production of recombinant proteins and antibodies,Cell therapy and tissue engineering,ect), we recommend using a monoclonal cell line.
-
3. Why is the timeline of stable cell line development longer?
The time required mainly depends on the screening of positive clones and further genetic stability testing for stable cell lines.
-
4. How are stable cell lines delivered?
Stable cell lines are shipped on dry ice to minimize the effect of temperature change.
